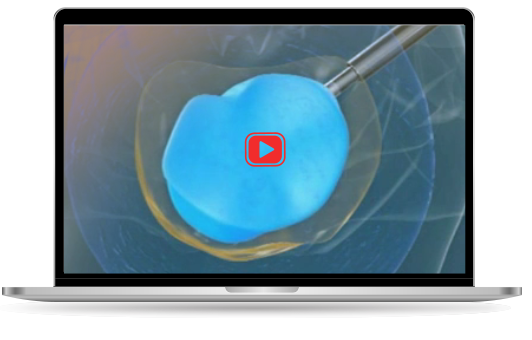
The Kunovus Disc Device aims to preserve the structural and biomechanical integrity of the lumbar intervertebral disc in patients undergoing a discectomy procedure
Chronic low back pain is frequently due to the defects or failures of the lumbar intervertebral disc, leading to herniation of the inner disc material. This can cause irritation and/or mechanical compression of the spinal cord or the exiting nerves, which results in pain and/or reduced mobility. The conventional surgical approach for treating lumbar disc herniation is discectomy, wherein the loose nuclear fragments in the spinal canal are removed and clearance of the nuclear cavity is performed. Although discectomy generally results in immediate pain relief, the operated disc may gradually collapse and become a source of recurrent pain. Recent data shows that one in five discs will re-herniate within the first decade of surgery, necessitating another surgical procedure. The Kunovus System and the Kunovus Disc Device have been developed to address this procedural lacuna and aims to preserve the structural and biomechanical integrity of the lumbar intervertebral disc after a discectomy procedure.

- Multiple US patents issued protecting the core technology
- Rigorous biomechanical testing on the device (in vitro testing, fatigue, aged-fatigue, static creep, shock, and expulsion testing)
- BioCompatibility and Toxicology studies completed per ISO 10993
- BioCompatibility Lapine study completed
- "Worst case scenario" Ovine studies completed
- Human cadaver study - validation of surgical techniques for device implantation
- Collaborations with leading spine surgeons and hospitals worldwide
Registered Office
6/22 Belgrave Street
Kogarah
Sydney, NSW 2217
Contact us
Call Us:
Fax: +61 2 8566 7177
PO Box 541, Kogarah 1485
Disclaimer: The Kunovus System and the Kunovus Disc Device are not approved in any jurisdiction for clinical use. The System and the Device are investigational only at this stage and not available for commercial sale.
NO ADVICE
This website contains general information about medical conditions and treatments. The information is not advice, and should not be treated as such.
LIMITATIONS OF WARRANTIES
The medical information on this website is provided “as is” without any representations or warranties, express or implied. Kunovus makes no representations or warranties in relation to the medical information on this website. Without prejudice to the generality of the foregoing paragraph, Kunovus does not warrant that: the medical or technical information on this website will be constantly available, or available at all; or the medical or technical information on this website is complete, true, accurate, up-to-date, or non-misleading.
PROFESSIONAL ASSISTANCE
You must not rely on the information on this website as an alternative to medical advice from your doctor or other professional healthcare provider. If you have any specific questions about any medical matter you should consult your doctor or other professional healthcare provider. If you think you may be suffering from any medical condition you should seek immediate medical attention. You should never delay seeking medical advice, disregard medical advice, or discontinue medical treatment because of information on this website.
LIABILITY
Nothing in this medical disclaimer will limit any of our liabilities in any way that is not permitted under applicable law, or exclude any of our liabilities that may not be excluded under applicable law.